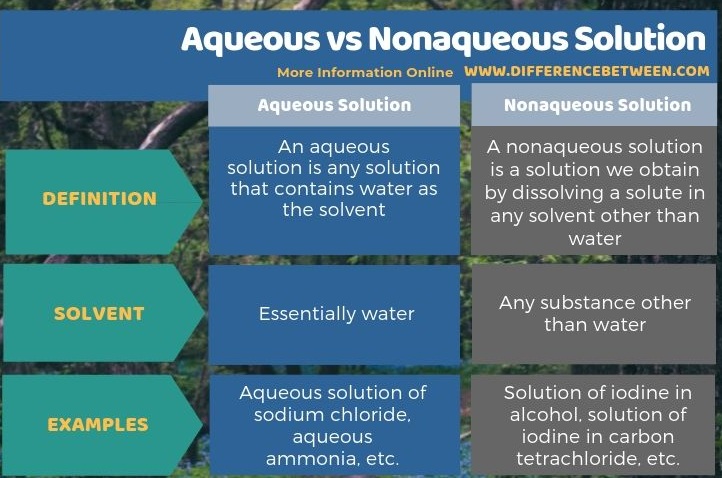
The key difference between aqueous and nonaqueous solution is that the solvent of an aqueous solution is water whereas, in nonaqueous solutions, the solvent is any substance other than water.
A solution contains a solvent and solute(s). The solutes are dissolved in the solvent. Here, the solutes and solvent should have the same polarity. Moreover, if the solvent is polar and solutes are nonpolar or vice versa, the solutes will not dissolve in the solvent, and we cannot obtain a solution.
CONTENTS
1. Overview and Key Difference
2. What is Aqueous Solution
3. What is Nonaqueous Solution
4. Side by Side Comparison – Aqueous vs Nonaqueous Solution in Tabular Form
5. Summary
What is an Aqueous Solution?
An aqueous solution is any solution that contains water as the solvent. Here, the solutes have to be hydrophilic and polar to dissolve in water to give an aqueous solution. Though we name water as the universal solvent, we cannot dissolve almost everything in it. For example, we cannot dissolve fat in water, so there are no aqueous fat solutions anywhere.
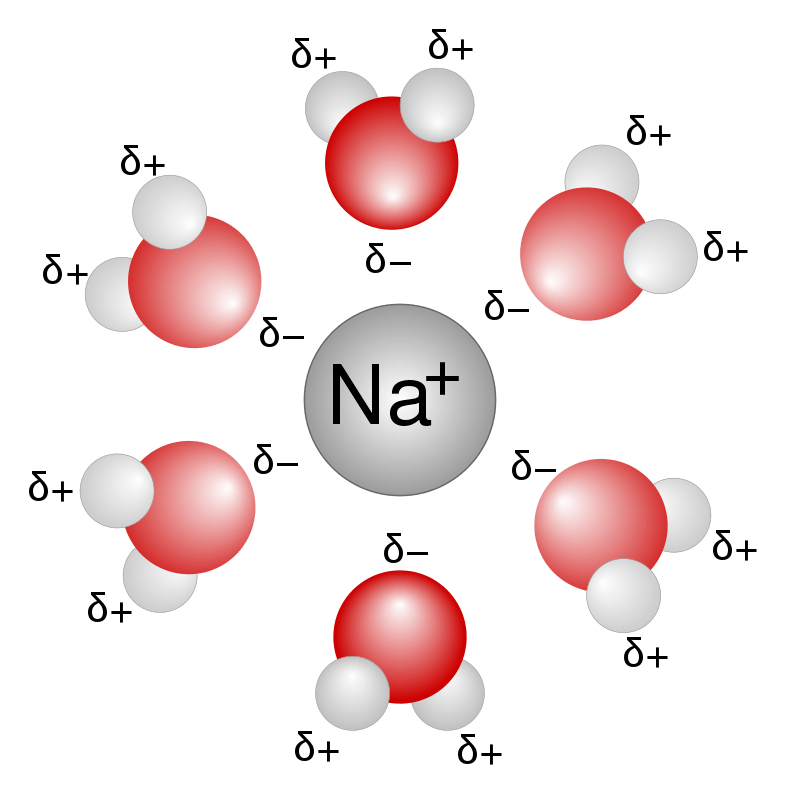
Figure 01: Sodium Ions in Water
When writing a chemical equation, we use the symbol (aq) as a subscript to indicate that substance is in an aqueous solution. If the solute can dissociate into ions upon dissolving in water, we say that aqueous solution is conductive because it can conduct electricity through the solution due to the presence of ions.
What is Nonaqueous Solution?
A nonaqueous solution is a solution we obtain by dissolving a solute in any solvent other than water. The solvent can be an organic compound such as acetone, toluene, ether, alcohol, benzene, etc.

Figure 02: Iodine in Alcohol
The solvent can be polar or nonpolar and depending on the polarity while solutes dissolve in the solvent. Solutions of iodine in alcohol and solutions of iodine in carbon tetrachloride are examples of nonaqueous solutions.
What is the Difference Between Aqueous and Nonaqueous Solution?
We can divide solutions into two groups as aqueous and nonaqueous depending on the solvent. The key difference between aqueous and nonaqueous solution is that the solvent of an aqueous solution is water, whereas, in nonaqueous solutions, the solvent is any substance other than water. Aqueous solutions of sodium chloride, aqueous ammonia, etc. are examples for aqueous solutions while solutions of iodine in alcohol, solutions of iodine in carbon tetrachloride, etc. are nonaqueous solutions.
Summary – Aqueous vs Nonaqueous Solution
Basically, we can divide solutions into two groups as aqueous and nonaqueous depending on the solvent. The key difference between aqueous and nonaqueous solution is that the solvent of an aqueous solution is water whereas, in nonaqueous solutions, the solvent is any substance other than water.
Reference:
1. Helmenstine, Anne Marie. “Aqueous Solution Definition in Chemistry.” ThoughtCo, Jan. 13, 2019, Available here.
Image Courtesy:
1. “Na+H2O” By Taxman – (Public Domain) via Commons Wikimedia
2. “J crow’s lugol’s iodine 2” By Amin – Own work (CC BY-SA 4.0) via Commons Wikimedia
ncG1vNJzZmivp6x7pbXFn5yrnZ6YsqOx07CcnqZemLyue8OinZ%2Bdopq7pLGMm5ytr5Wau26t0K6cqK2jYq6vsIynpqeZoaqysMHSZqqopKWptrC6jg%3D%3D