Ammonium Nitrate vs Urea
Compounds containing Nitrogen are commonly used as fertilizers because nitrogen is one of the highly essential elements for plant growth and development. Ammonium nitrate and urea are such nitrogen containing solids.
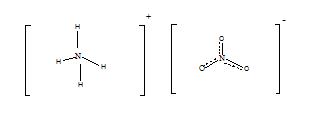
Ammonium nitrate
Ammonium nitrate has the chemical formula of NH4NO3. This is the nitrate of ammonia, and it has the following structure.
At room temperature and standard pressure ammonium nitrate exist as an odorless, white crystalline solid. This is an acidic salt with a pH of about 5.4. Its molar mass is 80.052 g/mol. Melting point of ammonium nitrate is about 170 °C and it decomposes when heated to about 210 oC. Ammonium nitrate is primarily used for agricultural purposes. It is rich in nitrogen, so it is used as a fertilizer, to supply nitrogen to plants. Since its direct contact with chemical is not hazardous and toxicity of it is less, it is beneficial to use as a fertilizer. Moreover, heating or ignition causes ammonium nitrate to explode. Therefore, it is used as an oxidizing agent in explosives. Because of this explosive nature, when storing ammonium nitrate we should be extra careful. Ammonium nitrate is stable, but when it is in the molten state risk for explosion is higher. The risk increases if it comes to contact with oxidizable materials such as oil, diesel, paper, rag, or straw. Production of ammonium nitrate is a simple chemical reaction. When nitric acid is reacted with ammonia liquid, ammonium nitrate in the solution form is produced. Industrially, concentrated nitric acid and ammonia gas are used for production. Since this is a highly exothermic and violent reaction, it is challenging to produce it in large scale. Being a salt, Ammonium nitrate is highly soluble in water. Therefore, when it is used as a fertilizer can be washed off and accumulates in water bodies. This could be a fatal condition for aquatic life.
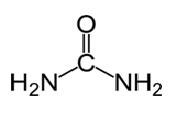
Urea
Urea has the molecular formula of CO(NH2)2 and the following structure.
It is a carbamide with the functional group C=O. Two NH2 groups are bonded to the carbonyl carbon from two sides. Urea is naturally produced in mammals in nitrogen metabolism. This is known as the urea cycle, and oxidation of ammonia or amino acids produce urea inside our bodies. Most of urea is excreted through kidneys with urine whereas some are excreted with sweat. The high water solubility of urea is helpful when excreting it from the body. Urea is a colorless, odorless solid, and it is non-toxic. Other than being a metabolic product, its main use is to produce fertilizer. Urea is one of the most common nitrogen releasing fertilizers, and it has a high nitrogen content compared to other solid nitrogenous fertilizers. In soil, urea is converted to ammonia and carbon dioxide. This ammonia can be converted to nitrite by soil bacteria. Further, urea is used to produce explosives like urea nitrate. It is used also as a raw material to produce chemicals like plastics and adhesives.
What is the difference between Ammonium Nitrate and Urea? • Molecular formula of ammonium nitrate is NH4NO3. Molecular formula of urea is CO(NH2)2. • Ammonium nitrate is a salt, whereas urea is not. It is a carbamide (organic molecule). • When dissolved in water ammonium nitrate produces an acidic solution. In contrast urea solutions are neither acidic nor alkaline. |
ncG1vNJzZmivp6x7pbXFn5yrnZ6YsqOx07CcnqZemLyue8OinZ%2Bdopq7pLGMm5ytr5Wau26tzKamp6GlonqvtdOrmK2dXZa7pXnVrGSuqpWWfA%3D%3D