The key difference between addition and substitution reaction is that the addition reaction is a chemical reaction that forms a large molecule from two or more small molecules whereas the substitution reaction is a chemical reaction in which atoms or functional groups replace the atoms or functional groups of a molecule.
Chemical reactions are the changes in the matter by chemical means. Addition reactions are combination reactions in which large molecules form from the combination of small molecules. Substitution reactions are replacement reactions in which moieties of molecules replace the moieties of other molecules. this results in different compounds.
CONTENTS
1. Overview and Key Difference
2. What is Addition Reaction
3. What is Substitution Reaction
4. Side by Side Comparison – Addition vs Substitution Reaction in Tabular Form
5. Summary
What is Addition Reaction?
Addition reactions are chemical reactions in which a large molecule forms from two or more small molecules. Here, byproducts do not form. Therefore, it is a very simple form of organic chemical reactions. we call the product “adduct”. These reactions are limited to alkenes and alkynes.
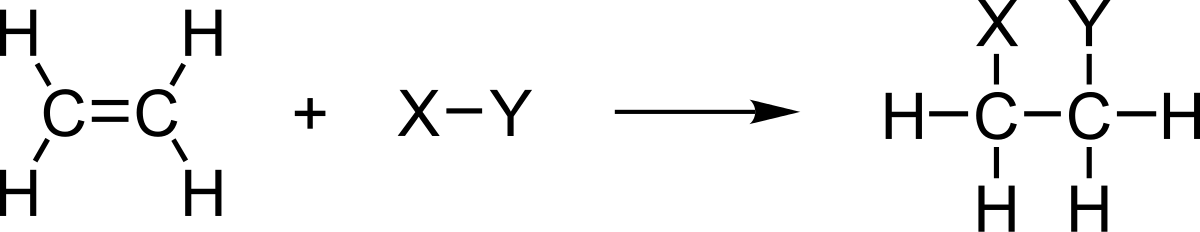
Figure 01: Ethene can undergo Addition Reactions
Moreover, carbonyl groups and imine groups also can undergo this type of reactions because of the presence of double bonds. Addition reactions are the opposite of elimination reactions. The two main types are electrophilic addition reactions and nucleophilic addition reactions. When these reactions cause polymerizations, we call it addition polymerization.
What is Substitution Reaction?
Substitution reactions are chemical reactions in which moieties of molecules replace the moieties of other molecules. These moieties can be either atom, ions, or functional groups. Most of the times, these reactions take place by replacing a functional group of a molecule with another functional group. Therefore, these are very important reactions in organic chemistry.
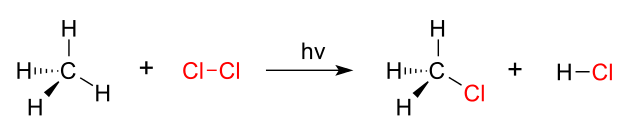
Figure 02: Methane Chlorination is a Substitution Reaction
There are two types of substitution reactions; namely, electrophilic substitution reactions and nucleophilic substitution reactions. Moreover, another category is there too; that is radical substitution reaction.
What is the Difference Between Addition and Substitution Reaction?
Addition reactions are chemical reactions in which a large molecule forms from two or more small molecules. Substitution reactions are chemical reactions in which moieties of molecules replace the moieties of other molecules. This is the main difference between addition and substitution reaction. As given below, there are more associated differences between addition and substitution reaction.
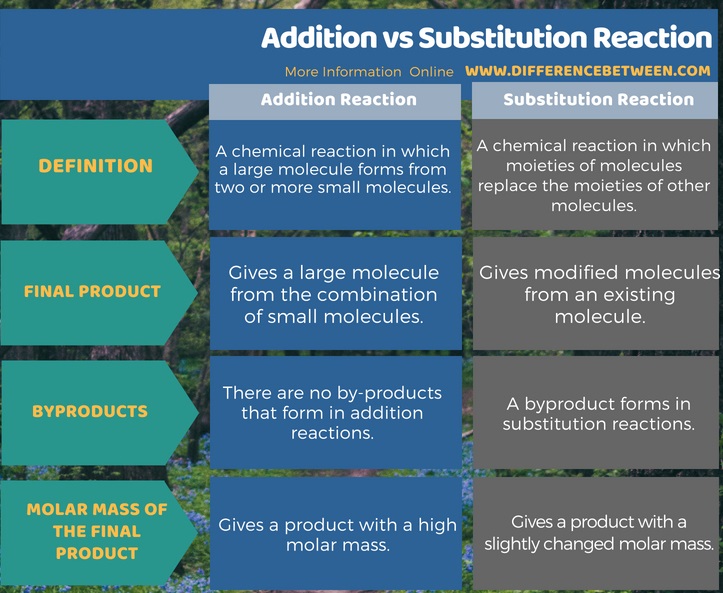
Summary – Addition vs Substitution Reaction
The two main important chemical reactions in organic chemistry are addition reactions and substitution reactions. The key difference between addition and substitution reaction is that an addition reaction is a combination reaction in which a large molecule forms from two or more small molecules whereas substitution reactions are chemical reactions in which atoms or functional groups replace the atoms or functional groups of a molecule.
Reference:
1. “Addition Reaction.” Wikipedia, Wikimedia Foundation, 27 June 2018.Available here
2. Helmenstine, Anne Marie. “Substitution Reaction Definition.” ThoughtCo, ThoughtCo. Available here
Image Courtesy:
1.’Addition reaction to ethene’By Calvero – Own work, (Public Domain) via Commons Wikimedia
2.’SubstitutionReaction’By V8rik at English Wikipedia, (CC BY-SA 3.0) via Commons Wikimedia
ncG1vNJzZmivp6x7pbXFn5yrnZ6YsqOx07CcnqZemLyue8OinZ%2Bdopq7pLGMm5ytr5Wau26tw52graGfo3qiusNmqq6ao6m2tcHToqanZaKarqTAyKilaA%3D%3D