The key difference between acidic salt and basic salt is that acidic salts form a solution that is less than pH 7.0 when dissolved in water, whereas basic salts form a solution that is higher than pH 7.0 when dissolved in water.
A salt is an ionic compound that contains a cation and an anion. It is a solid compound that has no net charge because the cations and anions combine with each other in such a way that the electrical charge of cations is balanced with that of anions. Depending on the ionic composition of a salt, the properties and reactivity can be determined. Therefore, we can categorize a salt into three groups as acidic salts, basic salts and neutral salts.
CONTENTS
1. Overview and Key Difference
2. What is an Acidic Salt
3. What is a Basic Salt
4. Side by Side Comparison – Acidic Salt vs Basic Salt in Tabular Form
5. Summary
What is an Acidic Salt?
Acidic salts are ionic compounds that can form acidic solutions upon dissolution in water. That means; the acidic salt forms an aqueous solution that is less than pH 7.0. This happens either due to the presence of a metal cation that can react as a Lewis acid or due to the presence of hydrolysable protons. Most commonly, acidic salts contain hydrolysable protons. These hydrolysable protons may exist in either the cation or the anion.
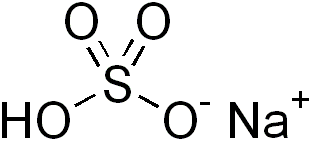
Figure 01: Sodium Bisulfite is an Acidic Salt
Hydrolysable Protons in Cation
Acidic salts that contain cations with hydrolysable protons are mainly ammonium ions. Ammonium ions originate from ammonium salts. Besides, these hydrolysable protons can occur in organic compounds that contain protonated amine groups. E.g. ammonium ion, methyl ammonium ion, ethyl ammonium ion, anilinium ion, etc.
Hydrolysable Protons in Anion
Acidic salts may contain the hydrolysable protons in the anion. Examples include bisulfite ion, dihydrogen citrate, bioxalate ion, etc. These anions contain protons that are weakly dissociated into water.
What is a Basic Salt?
Basic salts are ionic compounds that can form basic solutions upon dissolution in water. That means; these salts can form an aqueous solution having a pH higher than 7.0. Generally, a basic salt can deprotonate a water molecule and form hydroxide ions that can cause the basicity in the aqueous solution.

Figure 02: Sodium Sulfide is a Basic Salt
Some examples of basic salts include sodium bicarbonate, calcium carbonate, sodium acetate, potassium cyanide, and sodium sulfide. These salts can react with water, forcing the water molecules to remove a hydroxide ion.
What is the Difference Between Acidic Salt and Basic Salt?
The key difference between acidic salt and basic salt is that acidic salts form a solution that is less than pH 7.0 when dissolved in water, whereas basic salts form a solution that is higher than pH 7.0 when dissolved in water. Ammonium salts, sodium bisulfite, and calcium oxalate are some examples of acidic salts, while sodium bicarbonate, calcium carbonate, sodium acetate, potassium cyanide, and sodium sulfide are some examples of basic salts.
Below infographic summarizes the difference between acidic salt and basic salt.
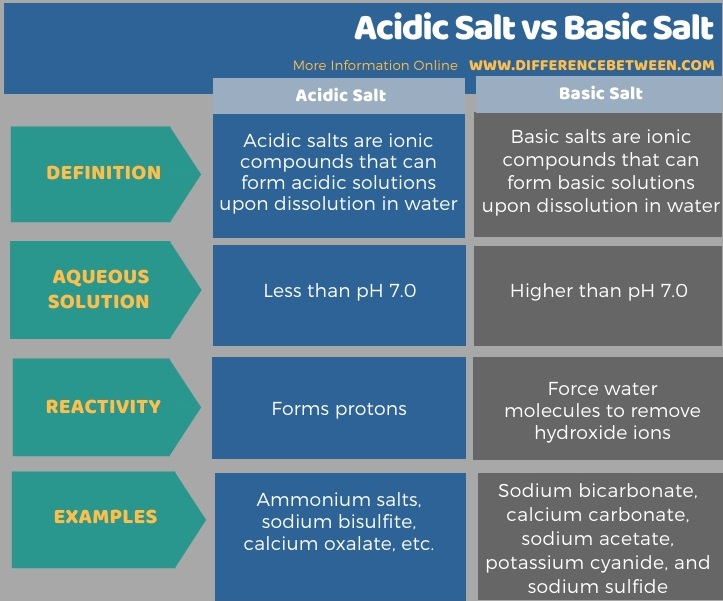
Summary – Acidic Salt vs Basic Salt
Depending on the ionic composition of a salt, the properties and reactivity can be determined. Therefore, we can categorize a salt into three groups as acidic salts, basic salts and neutral salts. The key difference between acidic salt and basic salt is that acidic salts form a solution that is less than pH 7.0 when dissolved in water, whereas basic salts form a solution that is higher than pH 7.0 when dissolved in water.
Reference:
1. “Acid-Base Properties of Salts.” Boundless Chemistry” Lumen, Available here.
2. “7.8: Acid-Base Properties of Salts.” Chemistry LibreTexts, Libretexts, 3 June 2019, Available here.
3. “Acid Salt.” Wikipedia, Wikimedia Foundation, 17 Oct. 2019, Available here.
Image Courtesy:
1. “Sodium bisulfate” By Edgar181 – Own work, Public Domain) via Commons Wikimedia
2. “Sodium sulfide nonahydrate crystals” By Leiem – Own work (CC BY-SA 4.0) via Commons Wikimedia
ncG1vNJzZmivp6x7pbXFn5yrnZ6YsqOx07CcnqZemLyue8OinZ%2Bdopq7pLGMm5ytr5Wau26twqKboptdqK6twIyapZ1lkpbAqq%2BMrJilrF8%3D